Market Authorization of Biological Medicinal Products
in EU
Definition of
Biological Medicinal Products:
According
to Part I Annex I of Directive
2001/83/EC, a biological
medicinal product is a product that contains a biological substance. A
biological substance is a substance that is produced by or extracted from a
biological source and that needs a combination of physico-chemical-biological
testing together with the production process and its control for its
characterization and the determination of its quality.
List of Biological
Medicinal Products:
|
1. |
Allergen products (e.g. for
allergy shots and tests) |
|
2. |
Blood or plasma derived
products and their recombinant alternatives |
|
3. |
Toxins |
|
4. |
Serums |
|
5. |
Monoclonal Antibodies |
|
6. |
Vaccines |
|
7. |
Biotechnology derived
proteins as active substances |
|
8. |
Advanced Medicinal Therapies
such as Tissue Engineering, Gene therapy, Cell therapy etc. |
Regulatory Pathway
for Biological Medicinal Products:
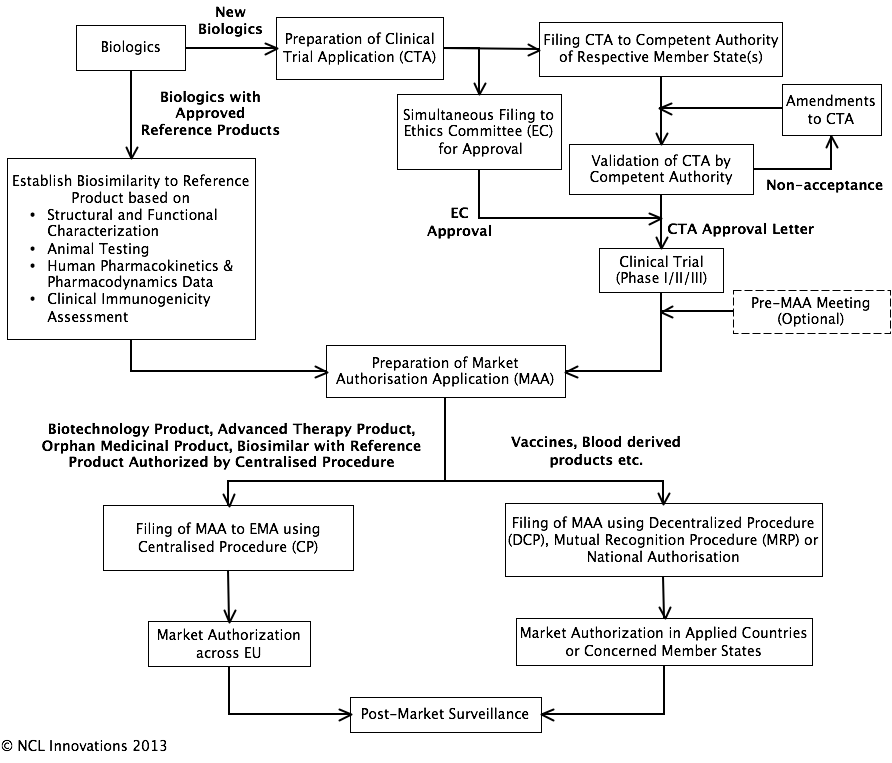
Market Authorization Procedures in EU:
|
Centralized Procedure |
National Authorization |
Mutual Recognition Procedure |
Decentralized Procedure |
|
This
procedure results in a Single Market Authorization that is valid in all EU
countries |
Individual
Application to Each Country in EU |
Single
Application Reviewed by One Member State for Authorization and other Member
States accept the decision |
Individual
Application to all Concerned Member States (CMS); Simultaneous review and
authorization by all CMS |
|
This procedure is mandatory
for following products:
3.
Orphan
medicinal products 4.
New
active substances for which the therapeutic indication is the treatment of: a.
Diabetes b.
Cancer c.
HIV/AIDs d.
Neurodegenerative
diseases e.
Auto-immune
diseases f.
Other
immune dysfunctions g.
Viral
diseases |
Applicable
for all products that fall outside the scope of EMA centralized procedure |
Applicable
for all products that fall outside the scope of EMA centralized procedure |
Applicable
for all products that fall outside the scope of EMA centralized procedure |
|
This procedure can be
optionally used for following products: 1.
Medicines
with significant therapeutic, scientific or technical innovation 2.
In the
interests of patients at community level: a.
Medicines
for pandemic b.
Generic
medicinal products of nationally authorized reference medicinal products c.
OTC
medicinal products d.
Generic
medicinal products of reference medicinal products authorized by this
procedure |
Overview of Contents of Clinical Trial Application (CTA):
1.
Covering
Letter
2.
Application
Form
a. Module 1 – Contains
information on the administration of the trial, trial site(s) with principal
investigator(s), the trial design and on the investigational medicinal products
(IMP).
b. Module 2 – Represents national or local Ethics
Committee application form (optional).
3.
Clinical
Trial Protocol
4.
Information
on Investigational Medicinal Products (IMP)
5.
Recruitment
Arrangments
6.
Subject information and the informed consent
procedure
7.
Suitability of the investigator and quality of the
facilities
8.
Insurance and indemnity
9. Financial
Arrangements
References:
[2] Guidelines on Application Format and Documentation for Clinical Trial
[3] Procedure for Market Authorization
[4] Procedure for Market Authorization – Centralized Procedure
[5] Presentation and Format of Dossier – Common Technical Document
[6] Guidelines for Similar Biological Medicinal Products