Market Authorization of Biological Products in USA
Definition of Biological Products:
According to USFDA, a biological
product means a virus, therapeutic serum, toxin, antitoxin, vaccine, blood,
blood component or derivative, allergenic product, protein (except any
chemically synthesized polypeptide), or analogous product, or arsphenamine or
derivative of arsphenamine (or any other trivalent organic arsenic compound),
applicable to the prevention, treatment, or cure of a disease or condition of
human beings. Biological products can be composed of sugars, proteins, or
nucleic acids, or a combination of these substances; or living entities, such
as cells and tissues. Biological products are made from a variety of natural
resources—human, animal, and micro- organism—and may be produced by
biotechnology methods.
List of Therapeutic Biological Products:
|
Regulated by CBER |
1. |
Allergenic extracts (e.g. for
allergy shots and tests) |
|
2. |
Blood, blood components,
plasma derived products |
|
|
3. |
Gene therapy products |
|
|
4. |
Antitoxins, antivenins, and
venoms |
|
|
5. |
Human tissue and cellular
products used in transplantation |
|
|
6. |
Vaccines |
|
|
Regulated by CDER |
7. |
Monoclonal antibodies |
|
8. |
Proteins (derived/recombinant) like Cytokines (e.g. Interferons), Enzymes (e.g. thrombolytics) etc. |
|
|
9. |
Growth factors (proteins that affect the growth of a cell) |
|
|
10. |
Immunomodulators (non-vaccine and non-allergenic products intended to treat disease by inhibiting or modifying a pre-existing immune response) |
|
|
11. |
Proteins intended for therapeutic use that are extracted from animals or microorganisms, including recombinant versions of these products (except clotting factors) |
Regulatory Pathway for Biological Products:
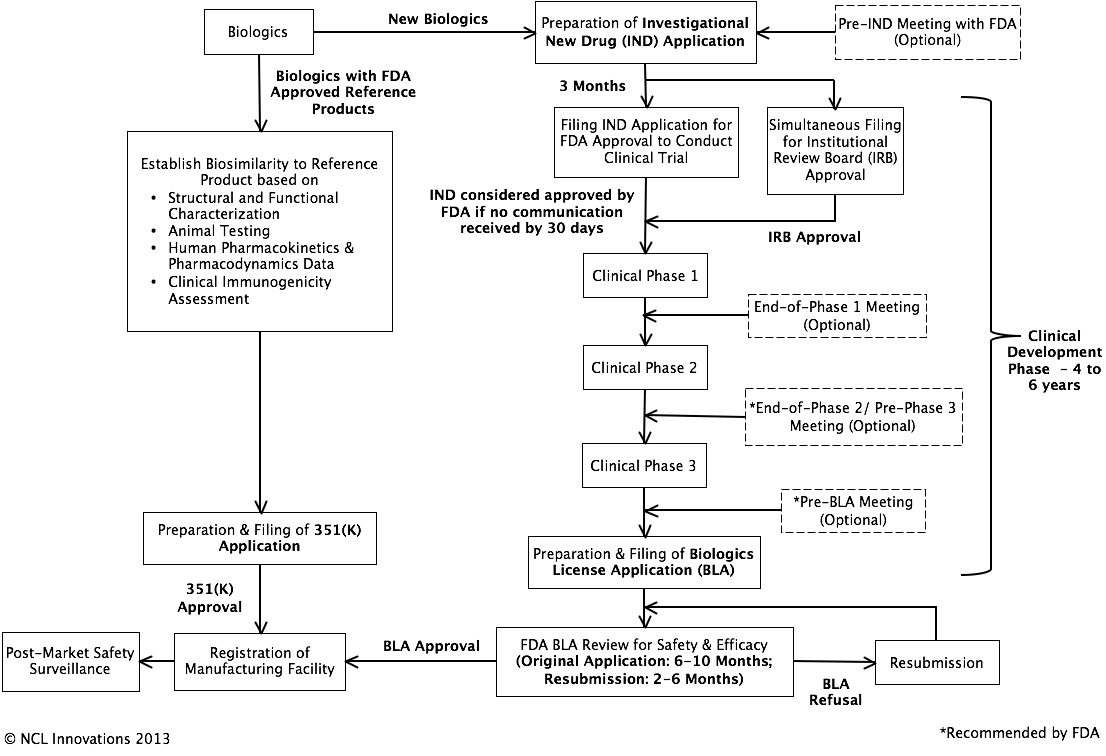
Overview of Contents of IND Application:
1. IND Form FDA 1571
2. Table of Contents
3. Introductory Statement
4. General Investigational Plan
5. Investigator’s Brochure and Form 1572
6. Protocol
a. Study Protocol and Design
b. Investigator Data
c. Facilities Data
d. IRS Data
e. CRO Statement and Details of person responsible for evaluating drug safety
7.
Chemistry,
Manufacture and Control (CMC) Data
a. General Drug Information (Nomenclature, Structure and Properties)
b. Manufacturer Details (Name and Address, Process, Control of Materials, Critical Process, Process validation, Manufacturing Process Development)
c. Characterization (Proof of Structure, Impurities)
d. Controls (Specification, Analytical Methods, Validation, Batch Analysis)
e. Reference Standards
f. Container Closure System
g. Stability Data
8. Pharmacology and Toxicology Studies Data
9. Previous Human Experience
10. Additional Information
References:
[1] Pre-IND Meeting
a.
Formal
Meetings Between the FDA and Sponsors or Applicants
b.
IND Meeting for Human Drugs
and Biologics
[2] IND Application
a.
Form 1571 – IND
Application
b.
Form 1572
– Statement of Investigator
d.
Fast Track Drug
Development Program
e.
Contents and
Format of IND for Phase I Studies
f.
INDs
for Phase 2 & 3 Studies
[3] Clinical Trials
a.
Early
Clinical Trials with Live Biotherapeutic Products
b.
FDA
acceptance of Foreign Clinical Studies not conducted under an IND
[4] Chemistry, Manufacturing
& Controls
a.
Comparability Protocol
- Protein Drug Products and Biological Products
b.
Analytical
Procedure & Method Validation
[5] Product Specific Guidance:
d.
Vaccines
[6] Biosimilar Products
a.
Scientific
Considerations in Demonstrating Biosimilarity to a
Reference Product
b.
Quality
Considerations in Demonstrating Biosimilarity to a
Reference Protein Product
c.
Implementation
of BPCI Act 2009
[7] BLA/351(K) Application
a.
BLA
Process
b.
Form 365h
– Application to Market
c.
Form 3674
– Certification of Compliance
[8] Post-Marketing Safety
Surveillance - http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/surveillance/ucm204091.htm
[9] Changes
to Approved BLA Application