Market Authorization of In-vitro Diagnostic Devices in
India
List of Notified
In-vitro Diagnostic Devices (IVDs):
1. IVD for HIV
2. IVD for HBsAg
3. IVD for HCV
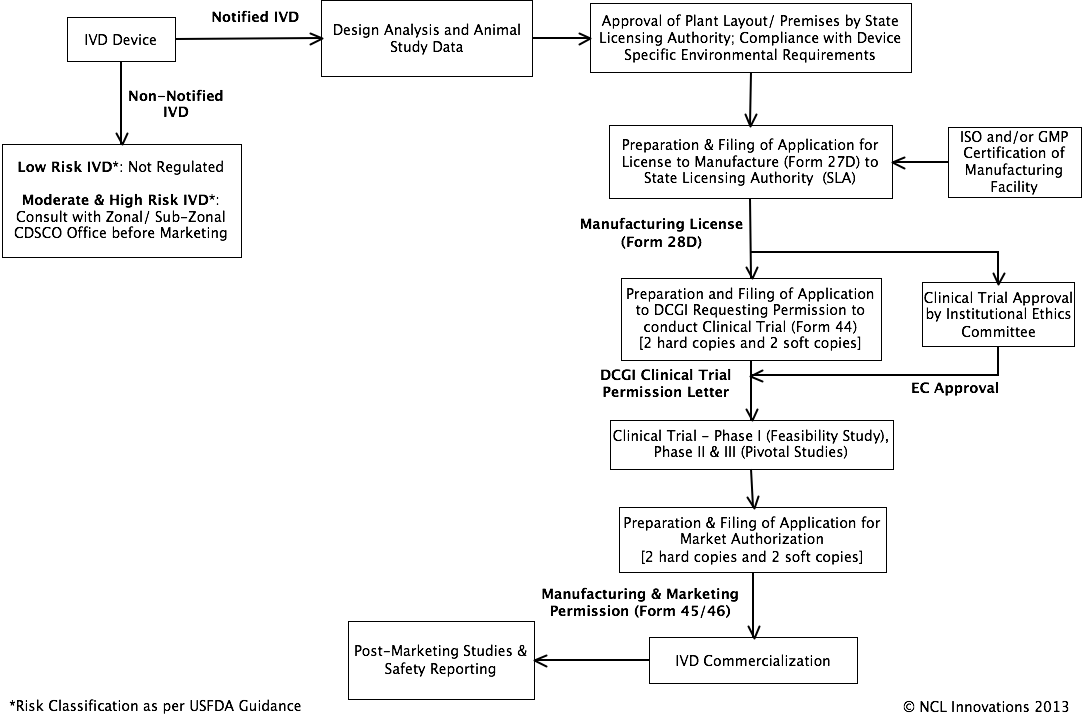
Regulatory Pathway
for IVDs:
References:
[1] The Drugs And Cosmetics Act And Rules, Department of Health, Government of India
[2] Guidance Document - Requirements for Conducting Clinical Trial(s) of Medical Devices in India
[4] Schedule M-IV_GMP for In-vitro Diagnostics