Market Authorization of Medical Devices in India
Definition of Medical
Devices:
According to the Drug and Cosmetics Act (DCA), a
medical devices are categorized as a drugs and defined as, “Such devices
intended for internal or external use in the diagnosis, treatment, mitigation
or prevention of disease or disorder in human beings or animals, as may be
specified from time to time by the Central Government by notification in the
Official Gazette, after consultation with the Board” [1].
List of Notified
Medical Devices:
|
1. Disposable Hypodermic Syringes |
|
2. Disposable Hypodermic Needles |
|
3. Disposable Perfusion sets |
|
4. IVD Devices for HIV, HBsAg and
HCV |
|
5. Cardiac Stents |
|
6. Drug Eluting Stents |
|
7. Catheters |
|
8. Intra Ocular Lenses |
|
9. I.V. Cannulae |
|
10. Bone Cements |
|
11. Heart Valves |
|
12. Scalp vein set |
|
13. Orthopedic implants |
|
14. Internal prosthetic replacements |
|
Additional Products: |
|
15. Blood Grouping Sera |
|
16. Ligatures |
|
17. Sutures |
|
18. Staples |
|
19. Intra Uterine Devices (Cu-T) |
|
20. Condoms |
|
21. Tubal Rings |
|
22. Surgical Dressing |
|
23. Umbilical Tapes |
|
24. Blood/Blood Component Bags. |
Regulatory Pathway:
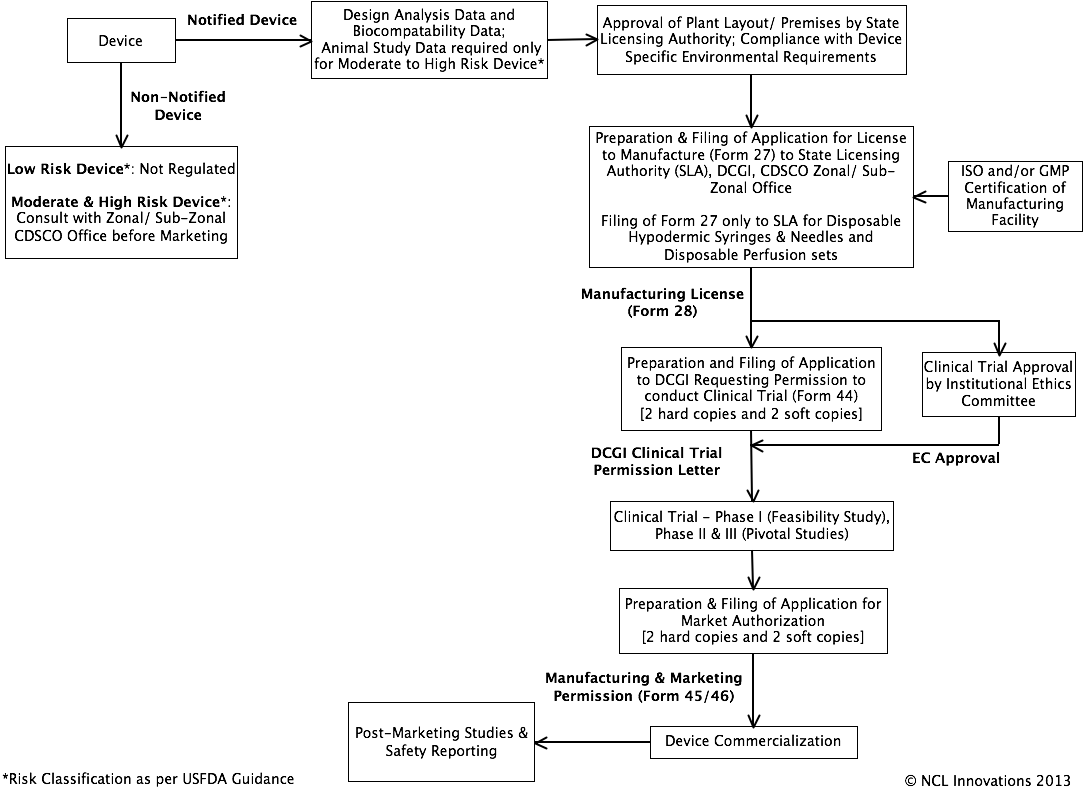
References:
[1] The Drugs And Cosmetics Act And Rules, Department of Health, Government of India
[2] Guidance Document - Requirements for Conducting Clinical Trial(s) of Medical Devices in India
[4] Schedule M-III_GMP for Medical Devices